General Physician | 6 min read
Everything you need to know about the COVID-19 vaccines in India
Medically reviewed by
Table of Content
Key Takeaways
- Covid-19 virus can overwhelm the immune system, wreaking havoc in the body
- The first Covid-19 vaccine in India was given on 16 January 2021
- Learn facts about the different types of Covid-19 vaccines in the country
Generally, your body's immune system can easily fight foreign pathogens and viruses and prevent you from falling sick, especially if you do not have any underlying condition compromising its strength. However, at times, a pathogen such as the Covid-19 virus can overwhelm the immune system, wreaking havoc in the body, causing serious illnesses and complications.
Vaccination is a primary preventive measure that helps the immune system recognize foreign microorganisms and prepare to eliminate or fight them if you are ever exposed to them. In simpler words, a vaccine teaches your immune system to battle pathogens – and this is the goal of the Covid-19 vaccine. It helps you develop antibodies, helping you better fight Covid-19, should you be exposed to the virus. Here are some key facts about Covid-19 vaccines in India.
What are the stages of vaccine development?
A vaccine undergoes six stages of development, and they are listed below.
Exploratory
In this primary stage, research is conducted to study the virus, how it attacks the human body, and the presence of antigens, such as a weakened strain of the virus, that can help fight the disease.
Additional Read: Ultimate guide for Covid-19Pre-clinical
In this phase, the immunity-building ability of the vaccine is tested on animals, tissue cultures and cell cultures. Most vaccines fail this stage, as they are unable to build immunity in the test subject.
Clinical trials
Here, the vaccine developer applies with governing bodies outlining the process of developing the vaccine, its effectiveness, and the immunization process. The governing bodies study the vaccine, and on approval, the vaccine must undergo the following three stages of human trials.
- Phase 1: Here, the vaccine is administered to less than 100 people, and its effectiveness and side-effects, if any, are studied.
- Phase 2: The vaccine is given to more than 100 people to study its safety, immunity-building capabilities, dosage, and schedule.
- Phase 3: Vaccine is given to a greater number of people to study the vaccine's effectiveness and any rare side effects.
- Approval: If the vaccine successfully passes through these stages, then the developer can gain approval.
- Manufacturing: Private pharmaceutical companies provide the infrastructure for mass manufacturing the vaccine.
- Phase 4:Once in the market, vaccine manufacturers will implement procedures to constantly monitor and assess the effectiveness and safety of the vaccine.
How was the Covid-19 vaccine developed so fast?
The entire procedure of vaccine development can take up to 10-15 years on average. However, the Covid-19 vaccine was developed in less than a year. This raised doubts and concerns about the effectiveness of the vaccine. However, this was largely possible due to global cooperation and funding. Further, SARS-CoV-2, the virus that causes Covid-19, is not a new virus and belongs to the coronavirus family that has caused major respiratory illnesses before. Moreover, significant progress in vaccine technology already existed.
Additional Read: Everything to know about COVID-19 careWhat was India's response to the Covid-19 pandemic?
On 30 January 2020, the WHO declared the coronavirus a public health emergency. The same day, India reported its first coronavirus case. As cases increased, on 24 March 2020, the Government declared a nationwide 21-day lockdown. In economic aid, the Government announced a Rs.1.7 trillion care package for the poor and migrant workers stranded in different states without employment. Moreover, the RBI announced a loan mortarium of three months. The lockdown was in effect till September, with exclusions made for migrant workers, the manufacturing sector, and the operation of various businesses. During the pandemic, the Government announced a Rs.20 trillion fiscal package to boost the flailing economy.
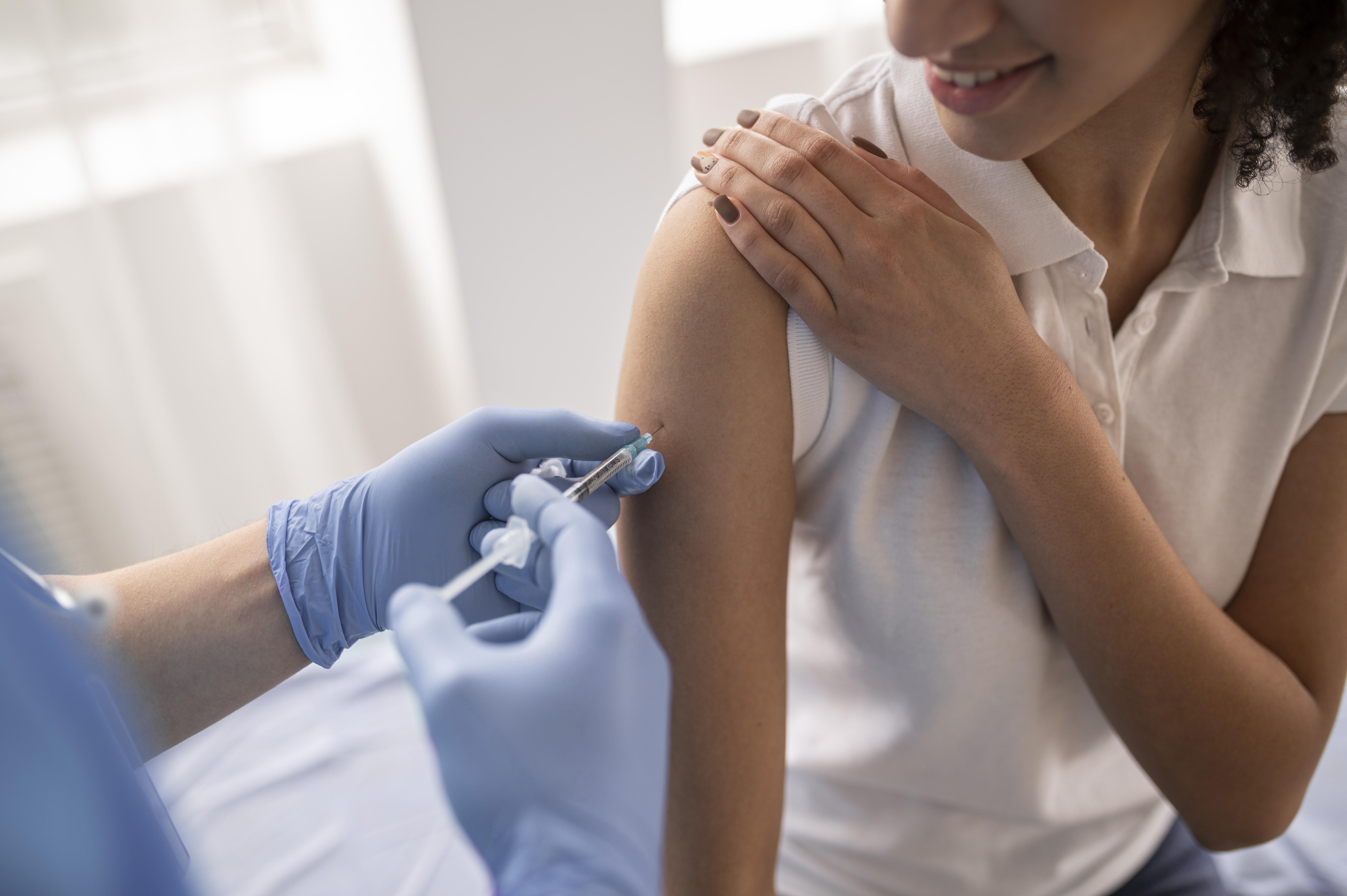
When was the first Covid-19 vaccine administered in India?
The first Covid-19 vaccine in India was given on 16 January 2021, kickstarting the vaccination drive, making it available to health and frontline workers. Over the months, the vaccination has been made available to other citizens, and so far, approximately 17 million Indians are completely vaccinated, receiving both doses. However, with current active cases standing at approximately 15 million, it is doubtful if the country will reach its goal of nationwide vaccination by July though it was the fastest country to reach 100 million doses.

What are the types of Covid-19 vaccine in the country?
Covaxin
Bharat Biotech, with a rich portfolio of 16 vaccines to date, developed India’s first indigenous Covid-19 vaccine – Covaxin. This is an inactive Covid-19 vaccine made using dead coronaviruses and has an efficacy of 81%. The immune system can still recognize the virus and is forced to develop antibodies to protect the body from the pandemic virus. It is administered in two doses, four weeks apart. Recently, the ICMR has declared that Covaxin neutralises against multiple variants of the Covid-19 virus and effectively neutralizes the double mutant strain.
Covishield
This Covid-19 vaccine, though developed by Oxford-AstraZeneca, is being manufactured by the Serum Institute of India. It consists of a common cold virus extracted from chimpanzees. In this Covid-19 vaccine, the common cold virus is made to look like the coronavirus, forcing the immune system to make antibodies that can fight the pandemic virus. This Covid-19 vaccine schedule consists of 2 doses, taken 4 to 8 weeks apart. The vaccine has an efficacy of ~63%, but with a longer gap between the two doses, the efficacy is reported to increase to 82-90%.
Sputnik V
The Russian-made Covid-19 vaccine, Sputnik-V, is similar to Covishield. The Indian Government has recently approved it. Sputnik-V has reported an efficacy of 92% as per research published in The Lancet, a renowned medical journal. This Covid-19 vaccine uses fragments of the coronavirus, delivered using a harmless common-cold-type virus, to prompt an immune response. This Covid-19 vaccine, unlike others, uses two different variations of the vaccines, injected 21-days apart. Using two different variations effectively is stated to provide a greater boost to the immunity system.
During a deadly second wave of the coronavirus, the Government of India has changed the Covid-19 vaccine eligibility to include everyone above the age of 18 from 1 May 2021. To date, 127 million vaccine doses have been administered. However, despite a promising start, the current second wave, in addition to the shortage of vaccines, has disrupted the vaccination drive, making the goal of vaccinating the entire country by July quite unachievable. Therefore, you must adhere to preventive measures, including staying at home, wearing a mask, washing your hands at regular intervals, and maintaining physical distance. Moreover, if you have a Covid-19 vaccine scheduled and are unnerved by some myths, lookup sound Covid-19 vaccine facts and dispel misinformation.
To access the right doctors in case you or a family member is concerned about Covid-19 vaccination, download the Bajaj Finserv Health app. This app allows you to book instant tele-consults with doctors so you can get medical help without leaving home and also comes with health plans to make healthcare more affordable. What’s you, you can use the app to track Covid-19 symptoms and even book a lab test. Download it today to explore its range of features and make healthcare a priority.
References
- https://www.who.int/news-room/q-a-detail/vaccines-and-immunization-what-is-vaccination?adgroupsurvey={adgroupsurvey}&gclid=Cj0KCQjw9_mDBhCGARIsAN3PaFNKnlNnuAy38Cy9E1eM6Y4tu4aHQStHiHtHy8Qj7pLEWURdSOA8UgYaAq7REALw_wcB
- https://onlinepublichealth.gwu.edu/resources/producing-prevention-the-complex-development-of-vaccines/
- https://www.ifpma.org/wp-content/uploads/2019/07/IFPMA-ComplexJourney-2019_FINAL.pdf
- https://indianexpress.com/article/india/covaxin-neutralises-double-mutant-strain-icmr-study-7282835/
- https://www.moneycontrol.com/news/business/companies/a-comparison-of-all-covid-19-vaccines-that-could-be-available-from-may-1-6791771.html
- https://www.businesstoday.in/coronavirus/covishield-90-effective-if-doses-given-after-gap-of-2-3-months-adar-poonawalla/story/435843.html
- https://www.who.int/news-room/feature-stories/detail/the-oxford-astrazeneca-covid-19-vaccine-what-you-need-to-know,
- https://www.thelancet.com/journals/lancet/article/PIIS0140-6736(21)00234-8/fulltext
- https://www.bbc.com/news/world-asia-india-56345591
Disclaimer
Please note that this article is solely meant for informational purposes and Bajaj Finserv Health Limited (“BFHL”) does not shoulder any responsibility of the views/advice/information expressed/given by the writer/reviewer/originator. This article should not be considered as a substitute for any medical advice, diagnosis or treatment. Always consult with your trusted physician/qualified healthcare professional to evaluate your medical condition. The above article has been reviewed by a qualified doctor and BFHL is not responsible for any damages for any information or services provided by any third party.